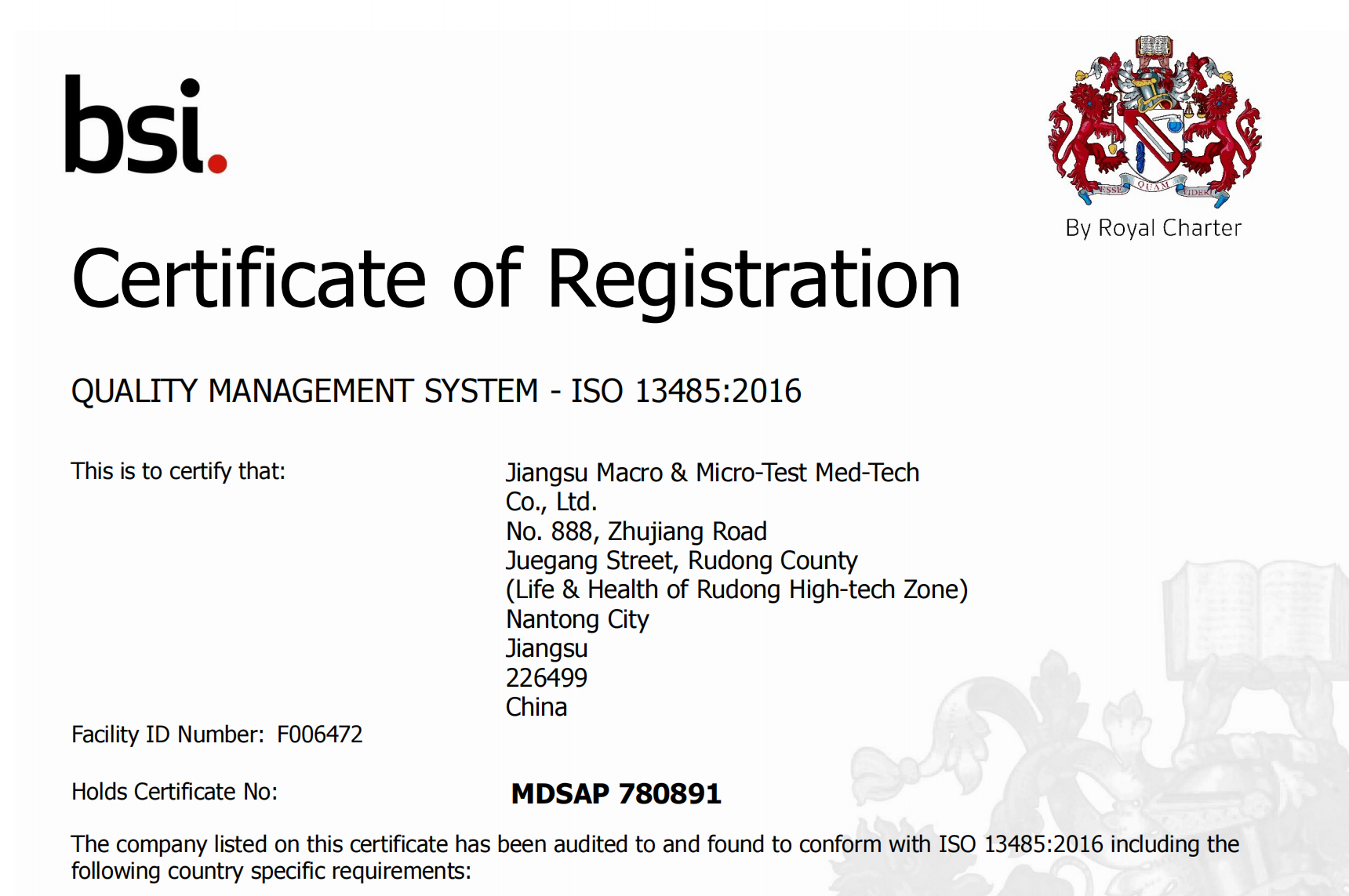
Isu tinofara kuzivisa kugamuchira kweMedical Device Single Audit Program certification (#MDSAP).MDSAP ichatsigira kubvumidzwa kwekutengeserana kwezvigadzirwa zvedu munyika shanu, kusanganisira Australia, Brazil, Canada, Japan neUS.
MDSAP inobvumira kuitwa kweongororo imwe chete yemugadziri wehugadziriso hwemhando yezvigadzirwa zvekurapa kuti igutse zvinodikanwa zvematunhu akawanda ekutonga kana zviremera zvinogonesa kutarisisa kwakakodzera kwemaitiro evagadziri vezvishandiso zvekurapa uku ichideredza kuremerwa kweindasitiri.Chirongwa ichi parizvino chinomiririra Australia's Therapeutic Goods Administration, Brazil's Agência Nacional de Vigilância Sanitária, Health Canada, Ministry of Health, Labor and Welfare and Pharmaceutical and Medical Devices Agency, uye US Food and Drug Administration's Center for Devices and Radiological Health.
Nguva yekutumira: Kubvumbi-13-2023